Background:
Primary Mediastinal B-cell Lymphoma (PMBL) comprises 10% of Diffuse large B-cell lymphoma (DLBCL), primarily affecting young adults. Combination chemoimmunotherapy with Rituximab, Cyclophosphamide, Doxorubicin, Vincristine and Prednisolone (R-CHOP) is effective, however up to 10-20% of patients may have either refractory disease or experience relapse within 12 months of completing treatment. Consolidative radiotherapy has often been used following R-CHOP, with retrospective data suggesting a survival benefit compared with R-CHOP alone. However, mediastinal radiotherapy increases risk of breast cancer and cardiovascular disease and the recently reported randomised IELSG37 study demonstrated no improvement in progression free survival (PFS) with the use of consolidative radiotherapy following chemoimmunotherapy. Dose-adjusted Etoposide, Prednisolone, Vincristine, Cyclophosphamide, Doxorubicin and Rituximab (DA-EPOCH-R) without radiotherapy has demonstrated excellent activity in a small phase II study but with increased chemotherapy toxicity. In IELSG37, PFS was inferior for patients treated with R-CHOP-21 compared with other chemoimmunotherapy regimens, supporting intensification of therapy beyond R-CHOP-21 in this entity. The outcome for patients with relapsed/refractory PMBL is poor, with response rates of around 25% to second line therapy, and a 2-year overall survival of 15%. Therefore, there remains a need to improve initial therapy for patients with PMBL.
Programmed-Death-1 (PD-1) ligands PD-L1/PD-L2 are commonly upregulated in PMBL. The PD-1 inhibitor Pembrolizumab blocks the interaction of PD-1 and PD-L1/2, and subsequent signalling, and has encouraging activity in relapsed/refractory PMBL, with an overall response rate of 43% (complete response 23%) and modest toxicity in a phase II study.
‘Window’ induction treatment involving delivery of checkpoint inhibitor therapy to patients with lymphoma, prior to initiation of chemotherapy has been investigated in phase II studies, showing encouraging efficacy and no safety concerns with this ‘chemotherapy-free’ initial treatment approach. The combination of Pembrolizumab and Rituximab is predicted to be synergistic, and in non-randomised trials in other B-cell lymphoma histologies, has demonstrated superior response rates to those expected with Rituximab monotherapy, suggesting synergism of the combination.
There is therefore compelling rationale for combining R-CHOP and Pembrolizumab as first-line therapy for PMBL. This study investigates the efficacy and safety of this novel, time-limited, radiotherapy sparing regimen.
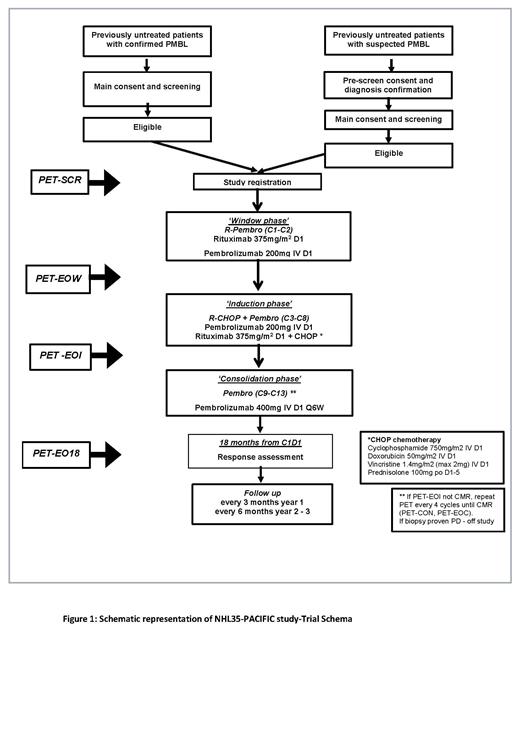
Methods ALLG-PACIFIC (ANZCTR- ACTRN12621001529831) is a phase II, single arm, open-label study of R-CHOP in combination with Pembrolizumab. The trial is open to recruitment across Australia. Approximately 35 patients with newly diagnosed PMBL, will be enrolled to receive two cycles of Rituximab (375mg/m 2) plus Pembrolizumab (200mg) once every 21 days ('window phase') followed by six cycles of R-CHOP plus Pembrolizumab once every 21 days ('induction phase') followed by five cycles of Pembrolizumab 400mg once every 42 days ('consolidation phase') (Figure 1). Adults (≥18 years) with treatment naïve histologically confirmed PMBL and adequate organ function are eligible. Key exclusion criteria are the presence of active autoimmune disease, requiring recent immunosuppressive therapy, life-threatening or organ-compromising lymphoma symptoms requiring urgent cytoreductive therapy, and other medically significant conditions that preclude eligibility to receive R-CHOP. Patients who require urgent cytoreductive therapy following initiation of window phase treatment may proceed immediately to induction phase chemotherapy.
The primary endpoint is 18-month event free survival. Key secondary endpoints include response to window phase treatment, overall survival, requirement for radiotherapy and safety of treatment including rates of early discontinuation due to treatment toxicity. Several biomarkers are also being investigated for their predictive value for treatment response including minimal residual disease status (measured by Adaptive Immunoseq and CAPP-Seq), PET-CT parameters (e.g., Metabolic tumour volume), and PD-1/PD-L1 expression and 9q24 alterations.
OffLabel Disclosure:
Lewis:Merck/MSD: Other: Advisory Board participant; Loxo/Lilly: Other: Travel, Accommodations, Expenses and Trial Steering Committee; Janssen: Honoraria; Roche: Consultancy, Honoraria; AstraZeneca: Consultancy, Honoraria. Giri:Royal Adelaide Hospital: Current Employment. Cochrane:Imago BioSciences, Inc., a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Research Funding; Beigene: Research Funding; Janssen-Cilag: Speakers Bureau. Francis:AIQ Solutions: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: current employment- Family member. Cheah:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; MSD: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Ascentage Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; AstraZenecca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Lilly: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG therapeutics: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Research Funding; Menarini: Consultancy, Honoraria; Genmab: Consultancy, Honoraria; Daizai: Consultancy, Honoraria.
Pembrolizumab is a type of targeted therapy drug called an immune checkpoint inhibitor (a type of immunotherapy). It is a monoclonal antibody that binds to the protein PD-1 on the surface of immune cells called T cells. It works by keeping cancer cells from suppressing the immune system. This allows the immune system to attack and kill the cancer cells. Many cancers, including PMBL, demonstrate up-regulation and over-expression of programmed-death-1 (PD-1) ligands PD-L1 and PD-L2. This is prominent in PMBL, and in this setting is largely due to genetic alterations at 9p24, including PD-L1/PD-L2 copy gain, amplification and translocations leading to the PD-L1/PD-L2 locus being placed next to the IgH locus. Binding of PD-L1/2 to PD-1 (it’s receptor on T-cells) provides a way for the tumour cells to evade the T-cell immunologic response, by negatively regulating T-cell mediated immune events. Inhibition of the PD1-L1/L2 pathway has been an effective treatment strategy across a range of malignancies. Within haematological malignancies, the most promising results have been noted in Hodgkin lymphoma and PMBL The PD-1 inhibitor pembrolizumab binds PD-1, therefore blocking PD-1 and PD-L1/2 interaction and subsequent signalling. Pembrolizumab has encouraging activity in patients with relapsed/refractory PMBL. In the phase II KEYNOTE-170 study, among 53 patients with relapsed/refractory PMBL and a median of 3 prior lines of therapy, the ORR was 43% (CR 21%) with median PFS 5.5 months. Toxicity was relatively modest, with grade ≥3 adverse events observed in 23% of patients, uncomplicated neutropenia being the most common.8 As of September 2020, pembrolizumab is subsidised on the PBS in Australia for patients with relapsed/refractory PMBL on the basis of these encouraging data.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal